Latest News
- SMARTDAC+ GX/GP/GM Release 3
- Release of Advanced Security Function (/AS) for SMARTDAC+ GM Series
- Dream Report – Advanced Reporting Software
SMARTDAC+ GX/GP/GM Release 3
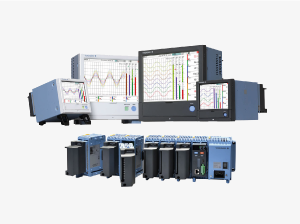
Yokogawa has launched release 3 of the SMARTDAC+® GX/GP series paperless recorders and GM series data acquisition system. This new release includes a number of new features and capabilities for the SMARTDAC+ system's GX series panel-mount type paperless recorder, GP series portable paperless recorder, and GM series data acquisition system.
With this release, four new firmware options and two new optional hardware modules (a pulse input module and a GM series DC power supply module) are available. The key features of the four firmware options are as follows:
- Heat treatment process option for Nadcap and SAE AMS2750E certification Nadcap and the SAE AMS2750E standard specify the pyrometric requirements for thermal processing equipment that are used in the heat treatment industry. In heat treatment processes where sensors, recorders, and data loggers are mounted directly on the heat treatment equipment to monitor their operating temperature, it is important for their calibration cycle to be clearly displayed. It is also important with accuracy tests for any calibration errors to be clearly displayed. In addition to having these capabilities, this heat treatment process option facilitates the management of calibration schedules. It can also set error compensation values for individual sensors, recorders, and data loggers, and record these values together with the measurement data. This option makes it easier for companies to obtain and maintain Nadcap and SAE AMS2750E certification.
- OPC-UA and SLMP communications options Release 3 includes a server option that complies with the OPC-UA communication standard necessary for Industry 4.0 automation. This enables measurement data stored on general-purpose data servers to be accessed directly by SCADA systems and HMIs. With this option, the GX/GP series paperless recorders and the GM series data acquisition system can function as subsystems for the transfer of data from field sensors and measuring instruments to upper level systems. Also available with release 3 is a seamless message protocol (SLMP*2) communications option that eliminates the need for a separate module or program to connect with SLMP-compliant PLCs via an Ethernet link.
- OPC-UA and SLMP communications options Release 3 includes a server option that complies with the OPC-UA communication standard necessary for Industry 4.0 automation. This enables measurement data stored on general-purpose data servers to be accessed directly by SCADA systems and HMIs. With this option, the GX/GP series paperless recorders and the GM series data acquisition system can function as subsystems for the transfer of data from field sensors and measuring instruments to upper level systems. Also available with release 3 is a seamless message protocol (SLMP*2) communications option that eliminates the need for a separate module or program to connect with SLMP-compliant PLCs via an Ethernet link.
- Multi-batch recording option Processes in the heat treatment, food/beverage, and pharmaceutical industries often require the use of more than one recording instrument to capture data on multiple batch processes. With the multi-batch option, a single recording device can record data from multiple batch processes, with the ability to individually control the start and stop of recording for each process. Furthermore, as SMARTDAC+ I/O units can connect to a network via Ethernet cables, access to multi-batch data distributed over a wide area is assured.
- Integrated drivers for Yokogawa recorders
- Automated report generation with full 21 CFR compliance
- Batch release including electronic signature function
- Alarm reporting
- Web portal for distribution / sharing of reports
Back to top
Release of Advanced Security Function (/AS) for SMARTDAC+ GM Series

Yokogawa has released a new advanced security function for their SMARTDAC+ GM series which provides secure and traceable data management. With this release, GM10 can now support the requirements of FDA regulation 21 CFR Part 11 for pharmaceutical, biotech, healthcare and medical device applications.
This latest GM/AS data acquisition system can be configured to utilise a three-way combination of user name, user identification codes and password to apply an electronic signature. Permissions can be further defined to provide a variety of access levels ranging from view-only access to full administrative and remote communication and configuration rights.
Further enhancements include a secure electronic data trail, maintaining records of all alarms, alarm acknowledgements, error messages (including unauthorised attempts at access), changes to configuration and calculation settings, and current user authorisation levels in binary files.
The SMARTDAC+ GM data acquisition system improves operational efficiency due to its modular design which facilitates the mounting and removal of modules. With modular I/O, users can scale their data acquisition system based on their process requirement, with the ability to upgrade as their needs evolve. The main unit contains memory for data acquisition and also supports SD cards for external storage.
Data acquisition systems are widely used in many industrial and test and measurement applications in a large variety of industries to acquire and record process data such as temperature, voltage, current, flow rate, and pressure for the evaluation testing of products, monitoring of plant equipment, and environmental monitoring.
Back to topDream Report - Advanced Reporting Software

Yokogawa is pleased to offer Dream Report; a web-enabled data integration and reporting software package designed for industrial and process automation. The software connects seamlessly with many of Yokogawa’s recorders and data acquisition products including SMARTDAC+ GX/GP, SMARTDAC+ GM, Daqstation DXA and Daqstation DX; collecting and archiving real-time data and providing online analysis. Dream Report automatically turns raw data into meaningful compliance, performance or maintenance professional reports, and can be configured to distribute them electronically.
The intuitive and easy interface allows fast project development with a low engineering cost. Dream Report is also able to access external history databases and proprietary archives and produce reports based on their historical data.
Suitable for a wide range of process and manufacturing applications, Dream Report also meets the stringent requirements of the Pharmaceutical and Biotech industries, providing audit trails, report version management, electronic signatures and pass/fail stamping, complying to the FDA’s 21 CFR Part 11 regulations. The main features include:
For more information, please contact uk.marketing@uk.yokogawa.com
Back to top




